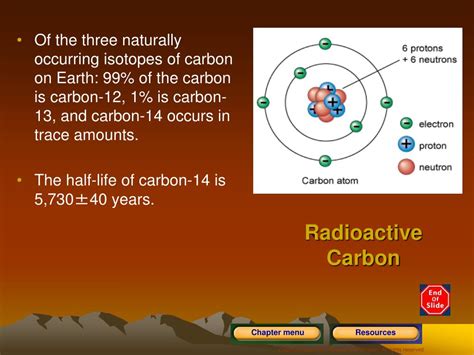
Carbon dating, a technique used to determine the age of organic materials, relies on the principles of radioactive decay. This method, developed by Willard Libby in the 1940s, has become a cornerstone in archaeology, anthropology, and geology, allowing researchers to accurately date specimens up to around 50,000 years old. The foundation of carbon dating lies in the unique properties of carbon-14, a radioactive isotope of carbon that is formed in the atmosphere when nitrogen-14 is struck by cosmic radiation. This process creates a small but consistent amount of carbon-14, which then mixes with the more abundant carbon-12 and is incorporated into living organisms through the food chain.
The key to carbon dating is understanding the half-life of carbon-14, which is approximately 5,730 years. Half-life is the time required for half of the amount of a particular radioactive isotope to decay. During this decay process, carbon-14 transforms into nitrogen-14, releasing a beta particle in the process. By measuring the amount of carbon-14 remaining in an organic sample, scientists can calculate how long ago the organism died, based on the premise that the sample contained the same amount of carbon-14 as the atmosphere at the time of its death. This assumption is grounded in the fact that all living organisms continuously exchange carbon with the atmosphere, maintaining a constant ratio of carbon-12 to carbon-14.
Key Points
- Carbon dating is based on the radioactive decay of carbon-14, with a half-life of approximately 5,730 years.
- The technique is used to date organic materials up to around 50,000 years old.
- Carbon-14 is formed in the atmosphere through the interaction of nitrogen-14 and cosmic radiation.
- The method assumes that the ratio of carbon-12 to carbon-14 in living organisms is constant and mirrors that of the atmosphere.
- Carbon dating has applications in archaeology, anthropology, geology, and other fields, providing valuable insights into the past.
Applications of Carbon Dating
Carbon dating has far-reaching implications across various disciplines. In archaeology, it helps in dating ancient civilizations, understanding cultural developments, and reconstructing historical events. For instance, the dating of the Dead Sea Scrolls and the Shroud of Turin has provided significant insights into their origins and authenticity. In anthropology, carbon dating is crucial for studying human evolution, migration patterns, and the development of societies. Geologists use carbon dating to study the age of rocks and fossils, shedding light on Earth’s history and the evolution of life.
Technical Specifications and Limitations
The accuracy of carbon dating depends on several factors, including the sample size, the presence of contaminants, and the equipment used for measurement. Traditional methods involve counting the beta particles emitted by the decay of carbon-14, using either gas proportional counters or liquid scintillation counters. However, these methods require relatively large samples and can be time-consuming. More recent techniques, such as accelerator mass spectrometry (AMS), allow for the direct counting of carbon-14 atoms and require much smaller samples, making them more versatile and efficient.
| Method | Description | Sample Size |
|---|---|---|
| Gas Proportional Counting | Measures beta particles emitted by carbon-14 decay | Several grams |
| Liquid Scintillation Counting | Detects light emitted when beta particles interact with a scintillator | Several grams |
| Accelerator Mass Spectrometry (AMS) | Directly counts carbon-14 atoms using a particle accelerator | Milligrams or less |
Evidence-Based Analysis and Future Directions
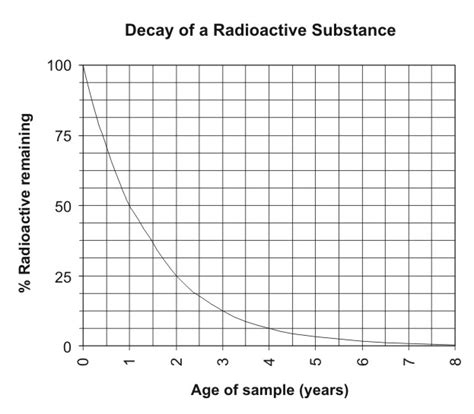
Despite its wide application, carbon dating is not without its challenges and limitations. Contamination of samples with modern carbon can lead to inaccurate results, and the technique cannot be used for inorganic materials or samples that are too old. Furthermore, variations in the Earth’s magnetic field and changes in the atmosphere’s carbon-14 levels over time can introduce uncertainties. To address these issues, researchers employ various techniques to purify samples and calibrate the carbon dating method against known age specimens, such as tree rings.
Looking forward, advancements in technology and methodology are expected to enhance the precision and applicability of carbon dating. The development of more sensitive detectors and the integration of carbon dating with other dating methods, such as uranium-thorium dating, will provide a more comprehensive understanding of the past. Additionally, the application of carbon dating in fields like environmental science, to study carbon cycling and climate change, underscores its versatility and the importance of continued research into the principles of radioactive decay and its applications.
What is the primary assumption of carbon dating?
+The primary assumption is that the ratio of carbon-12 to carbon-14 in living organisms is constant and reflects the atmospheric ratio at the time of the organism's death.
How does contamination affect carbon dating results?
+Contamination with modern carbon can lead to inaccurately young ages, while contamination with older carbon can result in ages that are too old. Therefore, careful sample preparation and purification are crucial.
What are some limitations of the carbon dating method?
+Limitations include the inability to date inorganic materials, samples older than approximately 50,000 years, and the potential for contamination and variations in atmospheric carbon-14 levels.
In conclusion, the application of radioactive decay in carbon dating has revolutionized our understanding of the past, enabling precise dating of organic materials across various disciplines. Through a deep understanding of the principles underlying this technique, including the formation of carbon-14, its half-life, and the assumptions of the method, researchers can navigate its limitations and continue to refine its applications. As technology advances and our understanding of Earth’s history evolves, the role of carbon dating in uncovering the secrets of the past will remain indispensable.