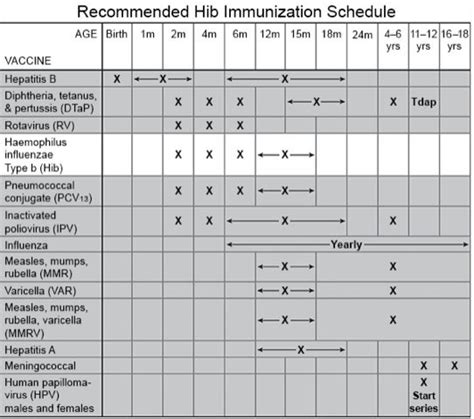
The Hib vaccine, also known as the Haemophilus influenzae type b vaccine, is a crucial component of the childhood vaccination schedule. Haemophilus influenzae type b (Hib) is a bacterium that can cause severe and potentially life-threatening diseases, such as meningitis, epiglottitis, and septic arthritis, primarily in young children. The introduction of the Hib vaccine has significantly reduced the incidence of these diseases. According to the Centers for Disease Control and Prevention (CDC), the Hib vaccine is approximately 95% effective in preventing invasive Hib disease.
Understanding the vaccine schedule is essential for parents and caregivers to ensure their children receive the necessary protection against Hib and other vaccine-preventable diseases. The typical Hib vaccine schedule involves a series of doses administered at specific ages, as outlined by the CDC and the American Academy of Pediatrics (AAP). The primary series of Hib vaccination usually consists of 2 or 3 doses, given at 2, 4, and in some cases, 6 months of age, followed by a booster dose at 12 to 15 months of age. This schedule may vary slightly depending on the vaccine product used and the child's health status.
Key Points
- The Hib vaccine is approximately 95% effective in preventing invasive Hib disease.
- The primary series of Hib vaccination typically consists of 2 or 3 doses, given at 2, 4, and sometimes 6 months of age.
- A booster dose is administered at 12 to 15 months of age.
- The vaccine schedule may vary depending on the vaccine product and the child's health status.
- Combination vaccines that include Hib, such as Pentacel (DTaP, IPV, and Hib) and Comvax (Hib and Hep B), are also available and may be used according to the CDC's recommended schedule.
Hib Vaccine Administration and Side Effects

The Hib vaccine is usually administered via intramuscular injection. Common side effects are mild and may include redness, swelling, or pain at the injection site, as well as fever. Serious side effects are rare. It’s essential for parents to discuss any concerns about vaccine side effects with their healthcare provider. In cases where children have certain medical conditions, such as a history of severe allergic reaction to a previous dose of Hib vaccine or to any component of the vaccine, the healthcare provider may recommend a different vaccination schedule or precautions.
Special Considerations for High-Risk Groups
Certain groups, such as children with immunocompromising conditions (e.g., HIV/AIDS, taking immunosuppressive drugs), might require special consideration regarding their Hib vaccination schedule. These children may be at higher risk for Hib disease and may need additional doses or a different type of vaccine. Healthcare providers will assess the individual’s risk factors and medical history to determine the most appropriate vaccination strategy.
| Vaccine Type | Dose Schedule |
|---|---|
| ActHIB (Hib) | 2, 4, 6 months, and 12-15 months |
| Pentacel (DTaP, IPV, Hib) | 2, 4, 6 months, and 12-15 months (booster dose may vary) |
| Comvax (Hib, Hep B) | 2, 4 months, and 12-15 months |

Evolution of Hib Vaccine Recommendations

Over the years, recommendations for Hib vaccination have evolved based on ongoing research and surveillance of Hib disease. For instance, the introduction of conjugate Hib vaccines in the late 1980s significantly improved the immune response in young children, leading to a dramatic decline in Hib disease incidence. Continuous monitoring and updates to vaccination schedules reflect the commitment to providing the most effective protection against vaccine-preventable diseases.
Vaccine Efficacy and Disease Surveillance
Efficacy studies and disease surveillance data play a critical role in informing vaccination policies. The near elimination of Hib disease in regions with high vaccination coverage rates is a testament to the vaccine’s effectiveness. However, in areas with low vaccination rates or where the vaccine is not widely available, Hib disease remains a significant public health concern. Global efforts to improve vaccine access and adherence to recommended vaccination schedules are vital to further reducing the burden of Hib disease worldwide.
What are the most common side effects of the Hib vaccine?
+Mild side effects may include redness, swelling, or pain at the injection site, as well as fever. Serious side effects are rare.
Can the Hib vaccine be given to children with weakened immune systems?
+Children with immunocompromising conditions may require special consideration. Healthcare providers will assess individual risk factors to determine the most appropriate vaccination strategy.
How effective is the Hib vaccine in preventing invasive Hib disease?
+The Hib vaccine is approximately 95% effective in preventing invasive Hib disease, according to the CDC.
In conclusion, the Hib vaccine is a crucial tool in preventing severe and potentially life-threatening diseases caused by Haemophilus influenzae type b. Adherence to the recommended vaccination schedule, as well as ongoing surveillance and research, are key to maintaining the significant public health gains achieved through vaccination. By staying informed and engaged, parents, caregivers, and healthcare providers can work together to protect young children from Hib disease and contribute to a healthier community.